

With molar concentrations, it is safe to assume that the solute is well mixed within the solution so that the concentrations change predictably with each dilution. Units like ppm are more common within microbiology when diluting bacterial cultures to low concentrations. Molarity is common for chemical applications.
#HOW TO DO DILUTION SERIES SERIAL#
A series of ten-fold dilutions is described as ten-fold serial dilutions. The concentration commonly reported in Molarity (M) or particles per ml (ppm). A ten-fold dilution reduces the concentration of a solution or a suspension of. The illustration above follows the relationship between the A 1:10 dilution is also called a 10x dilution. The final volume of the diluted sample is 1000 µL (1 mL), and the concentration is 1/10 that of the original solution. Mixing 100 µL of a stock solution with 900 µL of water makes a 1:10 dilution. For a 1:100 dilution, one part of the solution is mixed with 99 parts new solvent. For example, a 1:10 dilution is a mixture of one part of a solution and nine parts fresh solvent. They are described as ratios of the initial and final concentrations. Serial dilutions are often performed in steps of 10 or 100. The initial concentration and target range needed determines the size and number of dilution steps required. Serial dilutions are often performed in steps of 10 or 100. Doing this several times results in a range of concentrations. The diluted sample is then used as the base solution to make an additional dilution. Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.To perform a serial dilution, a small amount of a well-mixed solution is transferred into a new container, and additional water or other solvent * is added to dilute the original solution. Add 100 l of the 0.1 Tween 20 to 100 l of the diluted template to make a titration curve of six 2x serial dilutions. Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. Subsequently, one may also ask, what does serial dilution mean?Ī serial dilution is the stepwise dilution of a substance in solution.
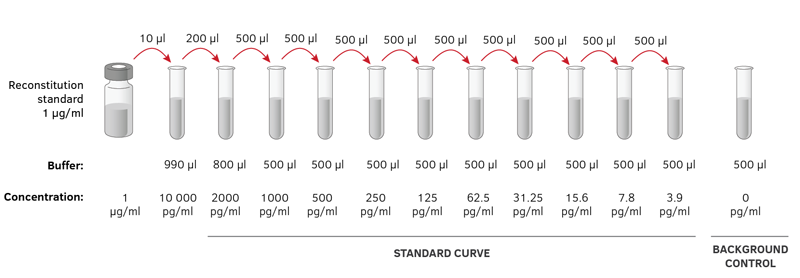
Each dilution will reduce the concentration of bacteria by a specific amount. Subsequently, question is, why is serial dilution important? A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale. Serial dilution is the stepwise dilution of a substance in solution. Likewise, what is serial dilution and why is it used?

A serial dilution is simply a series of simple dilutions which amplifies the dilution.
#HOW TO DO DILUTION SERIES HOW TO#
Each dilution will reduce the concentration of bacteria by a specific amount. Andersons How to Make Simple Solutions and Dilutions webpage). A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration.


 0 kommentar(er)
0 kommentar(er)
